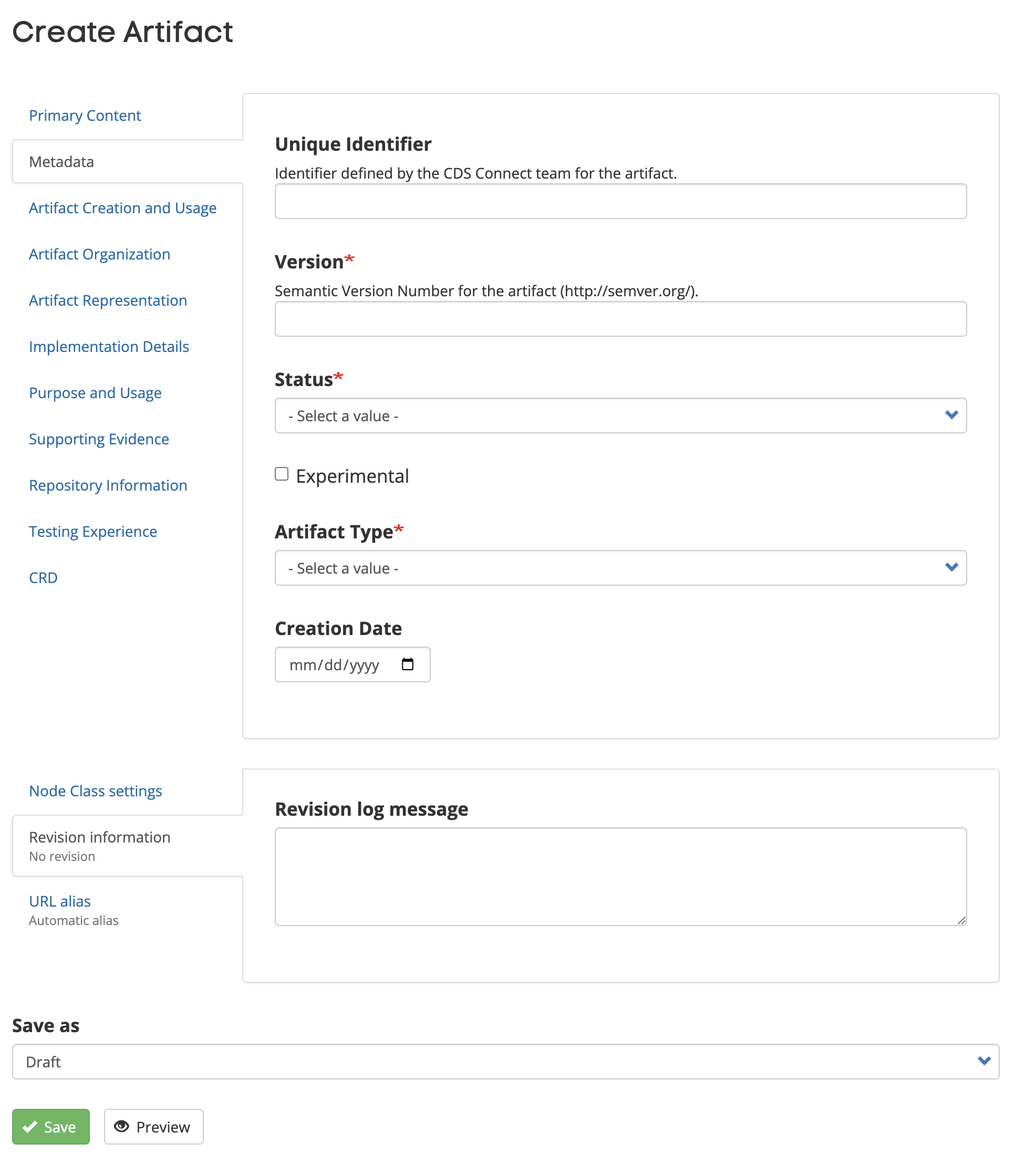
Metadata Tab
This content can help developers and implementors decide whether and how the artifact might match their own use case.
- Unique Identifier – Identifier defined by the CDS Connect team for the artifact.
- Version (Required) – The identifier that is used to identify this version of the artifact when it is referenced. Provide a version consistent with the Decision Support Service specification, use the format "Major.Minor.Revision" (e.g., 1.0.0). For more on Semantic Version Numbering, see http://semver.org/.
- Guidance: Use 1.0.0 for the initial public posting of the artifact. Subsequent updates to version numbers should be coordinated with the project team. For more on versioning CDS artifacts, see the March 2022 CDS Connect Work Group materials on the Community page.
- Status (Required) – The status of the artifact. See project FAQ page for further explanation.
- Active – Ready for normal use
- Draft – Under development, not ready for normal use
- Retired – Withdrawn or superseded, no longer of use
- Unknown – Status unclear
- Experimental – Select this checkbox, if applicable
- 1Experimental – “Yes” if this artifact is authored for testing purposes (or education/evaluation/marketing) and is not intended to be used in a clinical setting.
- Artifact Type (Required) – Select the type of the artifact from the dropdown. This value is used in the “Artifact Type” Simple Search feature. The following are the artifact types to select from:
- Reminder – Provides time-triggered information relevant to patient care
- Reference Information – Reference information to be used at the point of care
- Report – A report to provide additional value to clinicians or patients
- Risk Assessment – An assessment of the patient’s risk pertaining to a particular clinical topic
- Warning – Provides reactive information to address a possible error or hazard
- Smart Documentation Form – Provides fields that allow complete documentation of care
- Parameter Guidance – Proactive intervention to ensure diagnostic and therapeutic orders are complete and correct
- Order Set – A grouping of orders pertaining to a particular clinical context or workflow
- Data Summary – Provides a presentation of important data to optimize decision making
- Calculator – Performs mathematical calculations (e.g., a morphine milligram equivalent calculator, body mass index calculator)
- Event-Condition-Action (ECA) Rule – Monitor and react to situation to perform an action
- InfoButton – A mechanism for retrieving additional information based on contextual data
- Multimodal – Includes several different artifact “types” in one artifact (e.g., a documentation template, ECA rules, alerts, reminders)
- Alert – Provides event-driven (data-triggered) information
- Creation Date – Enter date manually or use calendar picker to add the artifact creation date. Authors currently select their own creation date and are encouraged to choose the date when the artifact is changed from “Draft” state to “Needs Review.”